Brief Narrative on Porosome Discovery & Cell Secretion Mechanism
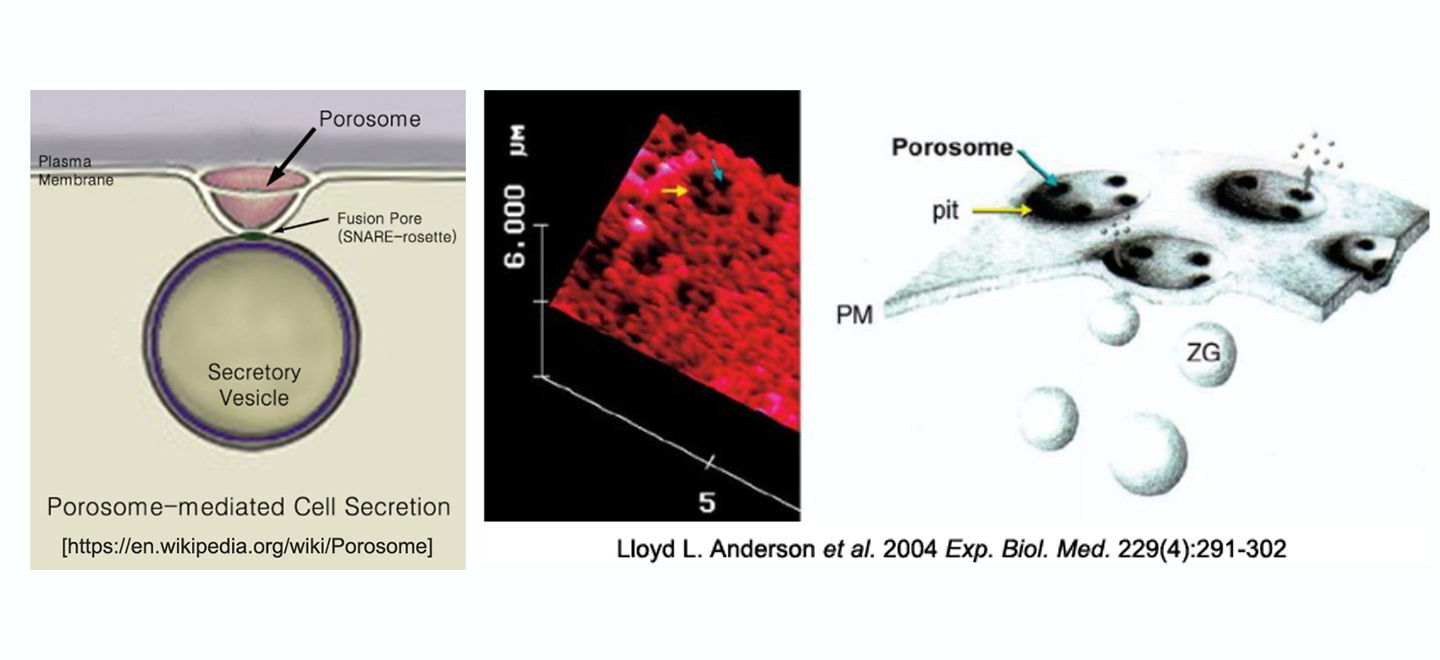
“Discovery of the porosome—the secretory portal at the cell plasma membrane—and its role in the process of secretion by cells, which is fundamentally important for physiology and medicine”
Summary: Secretion is a fundamental process through which cells communicate with their environment and exchange information in a multicellular context to reach homeostasis and sustain life. For decades, the prevailing worldview had been that secretion operates as an all-or-none event—‘complete fusion’—in which vesicles traffic to the cell surface, where the secretory-vesicle membrane fuses with and completely incorporates into the cell plasma membrane. The vesicle contents then diffuse away from the cell. This hypothesis, although attractive at first glance, had several key setbacks. First, it predicted a quantization of secretory products packaged into each secretory vesicle, when in fact, secretory vesicle size greatly varies even within the same cell, sometimes as much as 6-fold. Second, the level of additional regulation necessary to rapidly and precisely internalize and sequester vesicle-associated lipids and proteins following incorporation into the cell plasma membrane seem unnecessarily complex, given the tens of thousands of different membrane lipids and their differential distribution even between the same bilayer leaflets. Third, following a secretory episode, partially empty secretory vesicles accumulate within cells as observed in electron micrographs, demonstrating that secretory vesicles are capable of incomplete content release.
Bhanu P. Jena therefore hypothesized some 25 years ago, the presence of a tunable dial at the cell plasma membrane for secretory vesicle docking and fusion without full vesicle collapse, and the generation of hydrostatic pressure within vesicles to drive vesicular contents to the cell exterior. Through the use of atomic force microscopy, he identified nanoscale transmembrane cup-shaped lipoprotein structures, and named them ‘porosome,’ that have since been implicated in a wide range of secretory events. The family of proteins that make up the porosome has been biochemically identified and the mesoscale structure of the complex has been well characterized through atomic force microscopy, electron microscopy and solution X-ray methods. Defects in one or more porosome components have measurable, often highly potent, effects on the regulation of secretion, establishing links between point mutations and secretion-defective disease states such as cystic fibrosis that were previously correlative and are now causative. The discovery of the porosome solved the conundrum of fractional discharge of intra-vesicular contents from cells by providing an explanation for regulated graded secretion. Porosomes are cup-shaped supramolecular lipoprotein structures at the cell plasma membrane ranging in size from 15 nm in neurons, to 100-180 nm in endocrine and exocrine cells and composed of about 30–40 proteins. In comparison, the 120 nm nuclear pore complex is composed of nearly 1,000 protein molecules.
The discovery of the porosome, formation of SNARE ring complex at the porosome base to establish continuity between the porosome and the secretory vesicle membrane, and the molecular mechanism of secretory vesicle volume regulation, has brought about a clear and compelling understanding of the fractional intra-vesicular content release from cells during secretion. Additionally, it has raised new questions that the Jena laboratory and others continue to investigate: (a) What is the full set of cellular parameters regulating components of the porosome, and how do they act in concert to achieve a tuned output as a secretory portal? (b) Are porosomes serving multiple duties in cells beyond controlled fractional intra-vesicular release? (c) Are there different subclasses of porosomes where different secretory vesicles carrying different cargo dock and fuse? The impact and reach of the porosome discovery, and the associated findings ranging from understanding the fundamental cellular processes and the emergence of multicellularity, to detecting and preventing secretion-mediated disorders, have come to light.
Porosome Discovery & Cell Secretion Mechanism: Nearly 25 years ago, the Jena laboratory made the fundamental discovery of a new cellular structure called the ‘porosome’, and since, has elucidated its morphology, composition, function as the universal secretory machinery in cells, and has functionally reconstituted the porosome complex in lipid membrane and in live cells.
We can briefly summarize a time line of the discovery of the porosome and its participation in cell secretion: (a) the elucidation of the porosome structure, its chemical composition, and functional reconstitution into artificial lipid membrane and in live cells (4-14, 43-45); (b) the molecular assembly of membrane-associated t-SNARE proteins at the porosome base and v-SNARE proteins in the secretory vesicle membrane in a ring or rosette configuration resulting in the establishment of continuity between the opposing bilayers or ‘fusion pore’ formation in the presence of calcium (15-23,38); and (c) the molecular mechanism of secretory vesicle volume increase required for intra-vesicular content expulsion with great precision during cell secretion (24-30,42), provide a molecular understanding of the fractional release of intra-vesicular contents from cells during secretion. Consequently, publications from other laboratories on the porosome complex, and the involvement of various porosome proteins in secretion and in secretory defects (37,47-62), attest to the critical role played by the porosome in health and disease. It took nearly 20 years for the porosome to be included in textbooks, including the popular Medical Physiology textbook, co-edit by Walter Boron & Emile Boulpaep.
Since the 1950s, it was believed that secretory vesicles completely merge with the cell plasma membrane during secretion, resulting in release of the entire vesicular contents—an all or none mechanism. While this provides one mechanism for cell secretion, the observation of partially-empty vesicles in cells following secretion [Fig. 1] is incompatible with complete vesicle merger, suggesting the presence of an additional mechanism that allows partial discharge of intra-vesicular contents during secretion. In a Nature article [1993 Nature 363:497– 498], Erwin Neher wrote: “It seems terribly wasteful that, during the release of hormones and neurotransmitters from a cell, the membrane of a vesicle should merge with the plasma membrane to be retrieved for recycling only seconds or minutes later.” Jena hypothesized that the proposed mechanism involves a plasma-membrane structure, which would prevent the collapse of a secretory vesicle at the cell plasma membrane, instead enabling the vesicle to establish transient continuity with the cell plasma membrane, expel a portion of its contents, and disengage while remaining partially filled [Fig. 1A (see “√” mark), Fig. 1B]. This mechanism would enable the cell to retain the integrity of both the vesicle membrane and the cell plasma membrane.
The late Bruno Ceccarelli was a pioneer in the field of ‘transient’ or ‘kiss-and-run’ mechanism of secretory vesicle fusion at the cell plasma membrane enabling fractional release of intra-vesicular contents. Ceccarelli (1) proposed in 1973 the presence of such a process in cells. Then in 1990, Wolfhard Almers (2) hypothesized that the fusion pore (continuity established between the vesicle membrane and the cell plasma membrane), results from a “preassembled ion channel-like structure that could open and close”. In a later 1992 review, Julio Fernandez (3) opined that the difficulty in observing such channel-like structures, was the lack of ultrahigh- resolution imaging tools to monitor their presence directly and study their dynamics in live cells.
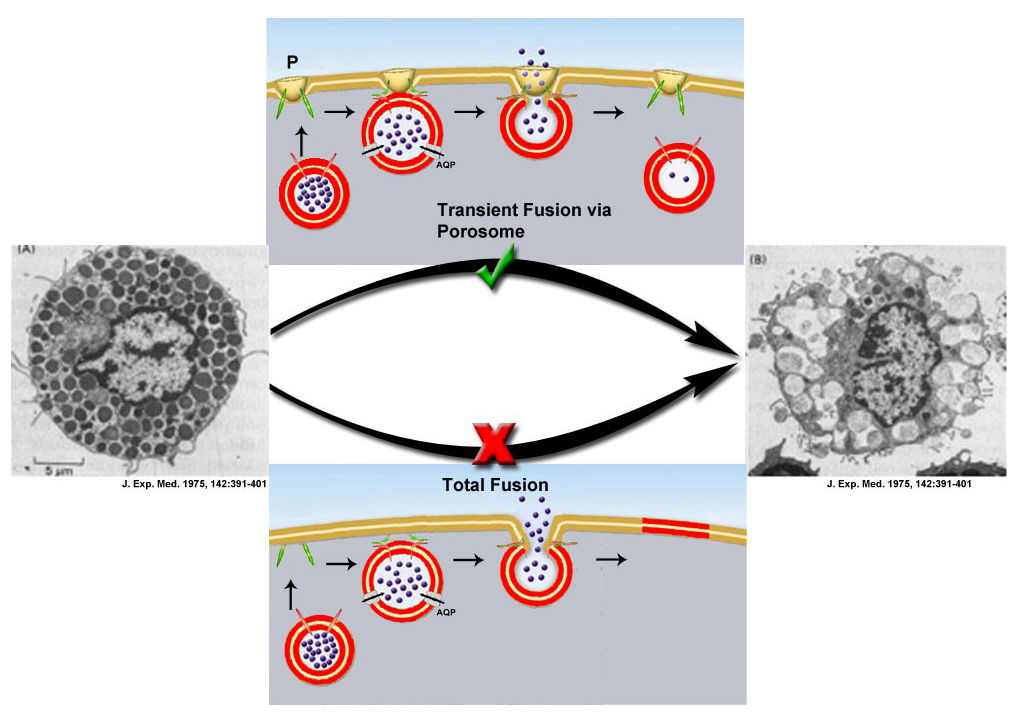
Fig. 1a. Electron micrographs of rat peritoneal mast cells, in resting (A, extreme left) and following secretion (B, extreme right). Note the fractional release of intravesicular contents following secretion (B) (J. Exp. Med. (1975) 142:391-401). This fractional release of intravesicular contents could only be achieved via the porosome (P)-mediated transient fusion mechanism shown (√).
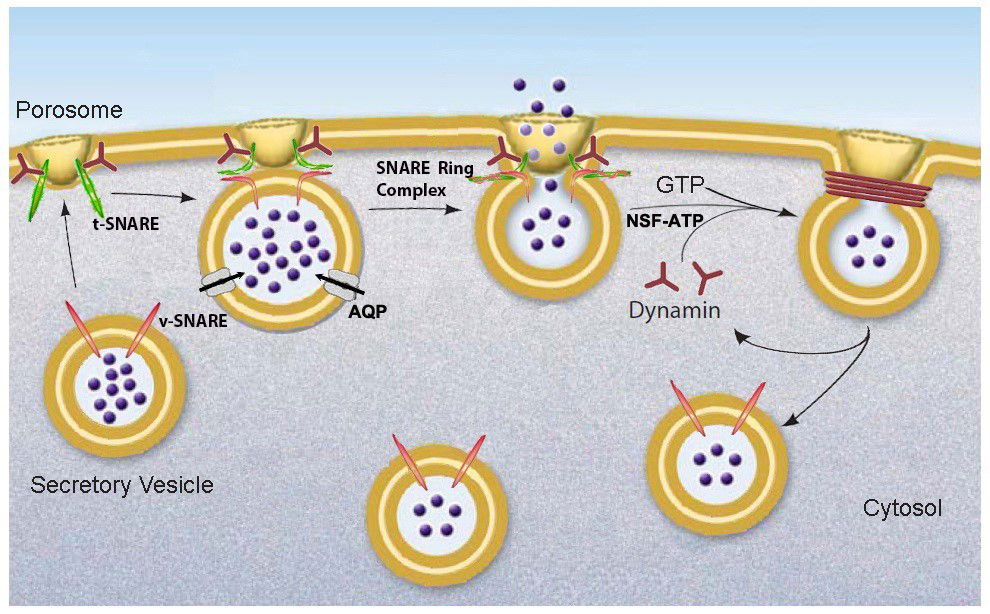
Fig. 1b: Schematic drawing of porosome-mediated fractional release of intravesicular contents from the cell during secretion. Secretory vesicles dock at the porosome base via t-SNAREs present at the porosome base and v-SNAREs present at the secretory vesicle membrane to form a t-/v-SNARE ring complex, establishing continuity between the opposing membranes [fusion pore] through which pressurized intra-vesicular contents [intra-vesicular pressure established via active transport of water through aquaporin or water channels (AQP) at the secretory vesicle membrane] are expelled to the outside during cell secretion. Following secretion, the t-/v-SNARE rosette complex is disassembled by NSF-ATP and the fused lipid membrane is cleaved by dynamin-GTP. The resultant partially empty vesicle then dissociates from the porosome at the cell plasma membrane.
In the mid-1990s, motivated by the goal of identifying cellular structures at the plasma membrane involved in the regulated fractional release of intra-vesicular contents from cells, Jena employed the then-newly-developed imaging modality of Atomic Force Microscopy (AFM) to study the morphology and dynamics of live pancreatic acinar cell surface at the nanometer scale during secretion. The major breakthrough came in 1995–1996, when Jena observed circular pit-like structures containing 100–180 nm depressions or pores [Fig 2A, B] at the apical plasma membrane of pancreatic acinar cells, where secretion is known to occur. During secretion, the depressions or pores grew larger, returning to their resting size following completion of secretion. Jena reported these results on January 1, 1997 in the Proceedings of the National Academy of Sciences (4). Five years later, new results from his laboratory established that the observed depressions are secretory portals at the cell plasma membrane (5,6). In January 2002 and February 2003, the Jena laboratory reported in two seminal studies, one in Cell Biology International (5), and the other in the Biophysical Journal (6), that following stimulation of cell secretion, gold-conjugated amylase antibodies (the starch-digesting enzyme amylase being a major intra-vesicular product secreted by the exocrine pancreas) accumulate at depressions, establishing depressions to be secretory portals in the cell (5,6) and the name ‘porosome’ was thereafter assigned to these structures. The study reported in the Biophysical Journal (6), further demonstrated the presence of t-SNAREs at the porosome base facing the cytosol, firmly establishing porosome structures to be secretory portals (6). Finally, using the conventional approach of electron microscopy (EM), Jena and colleagues obtained electron micrographs (see Fig 2, top left and right) of porosomes at the apical plasma membrane (PM) of pancreatic acinar cells with docked secretory vesicles called zymogen granule (ZG). In Fig 2A, the porosome membrane (POM, yellow arrowhead) associated with the ZG membrane (ZGM) is shown. In March 2002, the Jena laboratory in collaboration with Lloyd Anderson reported in Endocrinology (ref. 7, Fig 2 cover illustration lower right), the structure of porosome as well as porosome dynamics at the cell plasma membrane in growth hormone (GH) secreting cells of the pig pituitary gland, as well as the accumulation of GH-immuno-gold at porosome openings following secretion in these cells (7). The results further demonstrate porosome to be plasma membrane-associated secretory portals in cells. In the same year in a separate study, the Jena laboratory reported porosome structure-dynamics in chromaffin cells (8), and in September of 2003 (9) following isolation of porosome from acinar cells of the exocrine pancreas, and determination of its composition and reconstitution into lipid membrane, the porosome was demonstrated as the universal secretory portal in cells (9). In the same study (9), morphological details of the porosome complex associated with docked secretory vesicles was revealed using EM (9) [Fig. 2A]. Finally, in 2015–2016, isolated porosomes were functionally reconstituted in live insulin-secreting beta cells of the endocrine pancreas (10) by the Jena laboratory, establishing its critical function as the universal secretory machinery in cells and its promise in therapy.
In the past 20 years following the initial discovery of the porosome—and employing a combination of approaches such as AFM, biochemistry, electrophysiology, conventional EM, mass spectrometry, and SAXS— the Jena group and other laboratories have demonstrated and reported the presence of porosomes in all secretory cells examined, including perhaps, most significantly, neurons [Fig. 2C, D; Fig. 3] (11-14). Studies by Jena and his research team and those of other colleagues, establish this new cup-shaped supramolecular lipoprotein structure at the cell plasma membrane to be the secretory portals that performs the specialized task of fractional release of intra-vesicular contents from cells during cell secretion (12-14). Thus, the discovery of the porosome provides new understanding on how cells secrete, resulting in a paradigm-shift in our understanding of the secretory process in cells.
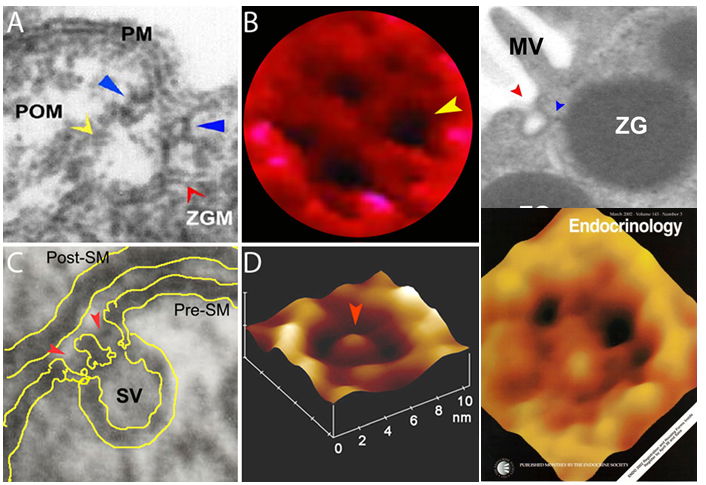
Fig. 2. Porosomes in the exocrine pancreas (A, B), neurons (C, D), growth hormone cell (Journal cover). (A) Electron micrograph of a single porosome at the apical plasma membrane (PM) of a pancreatic acinar cell showing the porosome membrane (POM, yellow arrowhead) associated with the membrane of a secretory vesicle called zymogen granule (ZGM). A circular ring structure (blue arrowhead) forms the neck of the porosome complex. (B) AFM micrograph of the apical surface topology of a live pancreatic acinar cell, demonstrating the presence of four openings or porosomes (one indicated by the yellow arrowhead). Porosomes in the exocrine pancreas range in size from 100-180 nm in diameter. (C) Electron micrograph of a neuronal porosome (red arrowheads) with a docked synaptic vesicle (SV) at its base, in the presynaptic membrane (Pre-SM) of the nerve terminal. Note the central plug in the porosome complex. (D) AFM micrograph of a neuronal porosome at the presynaptic membrane in an isolated synaptosome. Note the central plug (red arrowhead). The neuronal porosome is an order of magnitude smaller (10-17 nm in diameter) in comparison to the porosome in the exocrine pancreas. (Extreme Top Right) Electron micrograph of porosome (Fig 2 top left and right) next to a microvilli (MV) at the apical plasma membrane (PM) of a pancreatic acinar cell with docked secretory vesicle or ZG. (Extreme Lower Right) AFM micrograph of the apical surface topology of a live GH cell from pig pituitary demonstrating the presence of 100-180 nm in diameter porosomes (black circular openings). [Images obtained from: Proc. Natl. Acad. Sci. (1997) 94:316-321; Biophys. J. (2003) 85:2035-2043; Cell Biol. Int. (2004) 28:699-708; J. Microscopy (2008) 232:106-111; Endocrinology (2003) 143:1144-1148.
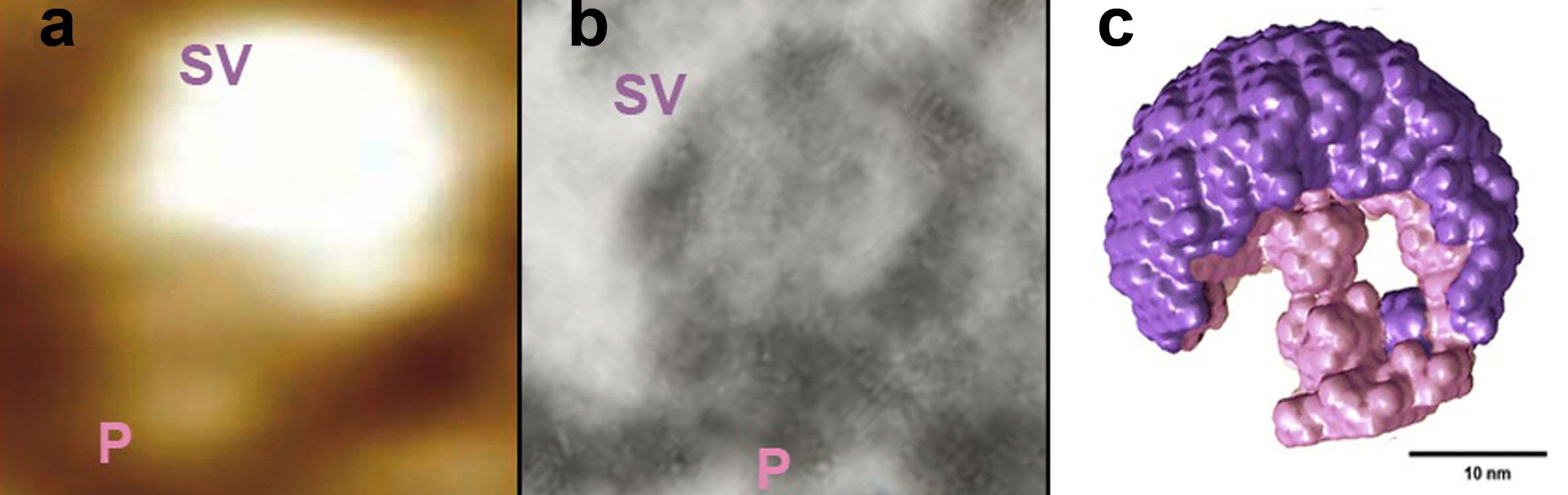
Fig. 3. Docked synaptic vesicles at the neuronal porosome in the presynaptic membrane of the nerve terminal, observed using AFM, EM, and small angle X-ray solution scattering (SAXS). (a) AFM micrograph obtained in fluid of a synaptic vesicle (SV) docked at the cup-shaped porosome complex (P) at the cytosolic compartment of the presynaptic membrane. Note the 35 nm SV docked to a 15 nm porosome complex. (b) An EM micrograph of a 35 nm SV docked to a 15 nm P at the presynaptic membrane [Cell Biol. Int. (2004) 28:699-708]. Note the central plug of the porosome complex in the EM micrograph. (c) The averaged SAXS 3-D structure of synaptic vesicle (purple) docked at the cup-shaped neuronal porosome complex (pink) at the presynaptic membrane in isolated synaptosome is presented (Bar = 10 nm) [Micron (2014) 56:37-43]. Note that AFM, EM, and SAXS, all demonstrate similarity in the docking and interaction of synaptic vesicles with the neuronal porosome complex.
The significance of the porosome discovery is reflected by the many publications on porosomes and associated transient fusion mechanism accompanied by fractional release of intra-vesicular contents from cells during secretion. In 2011 the porosome in hair cells was discovered by Dennis Drescher [Cell Biol. Int. Rep. (2011) 18:31-34]; in 2010 porosome in RBL-2H3 and BMMC cells were reported by Gang-yu Liu [J. Phys. Chem. B. (2010) 114:5971-5982]; porosomes in the exocrine pancreas have been further elaborated by the groups of Elshennawy in 2011[J. Am. Sci. (2011) 7:835-843]; Constantin Craciun in 2013 [Micron (2013) 44:137-142] and by the group of Mzia Zhvania [Micron (2012) 43:948-953, Fig. 4].
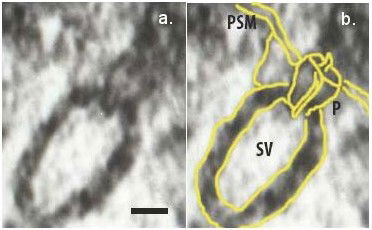
Fig. 4. Electron micrograph of docked synaptic vesicles (SV) at the base of a cup-shaped neuronal porosome complex (P) in the presynaptic membrane (PSM) of the nerve terminal, observed using electron microscopy. Micron (2012) 43:948-953.
Porosomes have also been discovered and extensively studies by the group of Márcia Attias in the unicellular pathogen Toxoplasma Gondii in 2011[J. Structural Biol. (2011) 177:420-430]; by Mzia Zhvania in normal and diseased cat and dog brain neurons in 2012 [Brain Res. Bull (2012) 87(2-3):187; Cell Tissue Biol. (2012) 6:69-72; Micron (2012) 43:948-953 [Fig. 4]; and by the group of Ilan Hammel and Isaac Meilijson in 2012 [J. R. Soc. Interface (2012) 9:2516-2526]. It has also been demonstrated in the exocrine, endocrine, and neuronal cells that “secretory granules are recaptured largely intact following stimulated exocytosis in cultured endocrine cells” [Proc. Natl. Acad. Sci. (2003) 100:2070-2075]; “single synaptic vesicles fuse transiently and successively without loss of identity” [Nature (2003) 423:643-647]; and “zymogen granule exocytosis is characterized by long fusion pore openings and preservation of vesicle lipid identity” [Proc. Natl. Acd. Sci. (2004) 101:6774-6779]. Similarly, in recent years, a great number of porosome-associated proteins such as chloride, calcium, and potassium channels, rab’s, SNAREs, dystrophin, dynamin, NSF, heterotrimeric GTP-binding proteins, GAPs, and myosin, among others have been implicated in secretion and diseases resulting from secretory defects (37,47–62).
Membrane fusion and secretory vesicle volume regulation for fractional release of intra-vesicular contents from cells: In the past two decades, the Jena laboratory has also contributed to our understanding of the fundamental molecular process involved in Ca+2 and SNARE-mediated membrane fusion (15–23), and on secretory vesicle volume regulation required for the regulated fractional release of intravesicular contents (24–30) via the porosome during cell secretion. This process enables cells to precisely regulate the discharge of a portion of their intra-vesicular contents during a secretory episode, while retaining full integrity of both the vesicle membrane and the cell plasma membrane.
Assembly of the native membrane-associated t-/v-SNARE complex and membrane fusion: In 1988 Richard Scheller discovered a secretory vesicle associated membrane protein (VAMP-1 or v-SNARE; ref. 31), and then in 1992 he and his team discovered another important protein present in the plasma membrane called syntaxin. Syntaxin is one of the two target SNARE or t-SNARE proteins (32). Then in 1989, Michael Wilson discovered SNAP-25, the other t-SNARE protein (33). Understanding the properties of the three SNARE proteins in membrane fusion requires a molecular understanding of their interactions, with the different SNARE proteins being present in opposing membranes (VAMP-1 in secretory vesicle membrane, and syntaxin and SNAP-25 in the cell plasma membrane). Because SNAREs are membrane-associated proteins, crystals of membrane-associated SNARE complex are required for X-ray crystallography. This has not been possible due to solubility problem of such membrane proteins. To circumvent issues associated with the solubility of membrane associated SNAREs, Axel Brunger and Reinhard Jahn in 1998 truncated the hydrophobic membrane anchoring domains of syntaxin and VAMP, to obtained crystals of a non-membrane associated t-/v-SNARE complex. Utilizing X-ray crystallography, Brunger and Jahn determined the atomic structure of the soluble SNARE complex at 2.4Å, which they reported in Nature (34). It was unclear however, whether the structure of the resolved soluble SNARE complex was identical to the native membrane-associated SNARE complex.
To address this issue, the Jena laboratory carried out high-resolution AFM studies combined with electrophysiological measurements. In a study reported in the Biophysical Journal in 2002 (15), they demonstrated that in the absence of membrane association, SNAREs fail to appropriately interact with each other or establish continuity between the opposing bilayers in presence of calcium. The Jena group further demonstrated for the first time that VAMP-1 proteins present in one membrane interacts with syntaxin and SNAP-25 proteins present in an opposing membrane, to assemble in a rosette or ring configuration, establishing continuity between the opposing bilayers in the presence of calcium (15-23). While it had been hypothesized that the interaction between t-SNAREs and v-SNARE present in opposing bilayers form rosette or ring structures (35), structural studies using high resolution AFM confirming this was first reported by Jena in the 2002 in the Biophysical Journal (15) [Fig. 5], and further established using high resolution EM (18-23). This SNARE rosette arrangement between opposing bilayers during membrane fusion is now widely accepted as the fundamental structure of the t-/v-SNARE complex associated with membrane fusion and cell secretion, and is widely published (36,37).
Role of Ca2+ in membrane fusion: In the 1970’s, the late Demetrious Papahadjopoulos had proposed the involvement of inter-membrane Ca2+-phospholipid complex in the fusion of opposing lipid membranes (38). To determine the involvement of Ca2+ in membrane fusion at the atomic level, the Jena group performed X-ray diffraction studies involving t- and v-SNARE reconstituted liposomes (16). Results from this study demonstrated that SNAREs overcome the repulsive forces between the opposing negatively charged lipid membranes to bring them within a distance of 2.8Å (16). They therefore concluded that if calcium was involved in the bridging of opposing bilayers via oxygen of the phospholipid head groups, calcium must be present at the site where the t- SNARE vesicles and v-SNARE vesicles make contact. t-SNARE vesicles and v-SNARE vesicles associated in the absence of calcium, would therefore fail to establish continuity between the opposing bilayers since hydrated calcium (with 6 water molecules surrounding it) measuring nearly 7Å would be unable to fit within the 2.8Å spacing separating the two opposing membranes. In 2004, this hypothesis was tested and confirmed experimentally by the Jena laboratory (17).
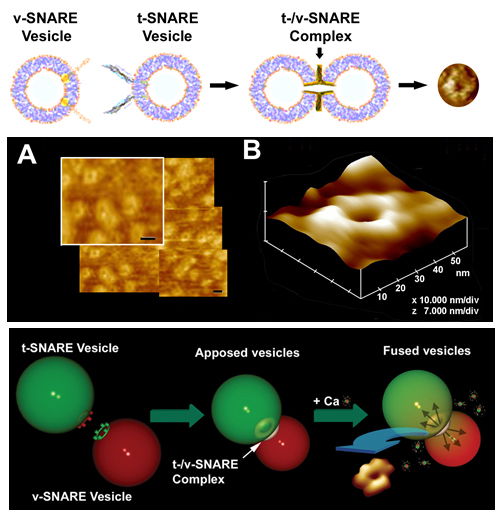
Fig. 5. Actual t-/v-SNARE ring complexes or rosettes formed following the interaction between membrane-associated t-SNAREs and v-SNAREs. Top panel is a schematic drawing depicting the interaction between t-SNAREs and v-SNAREs in opposing vesicles. AFM micrographs of the actual SNARE complex rings or rosettes are presented in the top right panel, in the middle panel, and in the right lower panel (15,16).
From these results, Jena further hypothesized that, following bridging of the opposing phospholipids by hydrated Ca2+, the loss of coordinated water associated with Ca2+ as well as those associated with the oxygen atoms of the phospholipid head groups must result in local dehydration, lipid mixing, and membrane fusion. In collaboration with Jeff Potoff, Jena tested this hypothesis using blind molecular dynamic simulations involving dimethyl phosphate (DMP), calcium, and water molecules (14). Confirming this hypothesis, results from the study demonstrated that hydrated Ca2+ is capable of bridging phospholipid head groups, and that this process results in the expulsion of water from both phospholipid head groups and Ca2+ (39). The simulation further demonstrated that the distance between the anionic oxygen in DMP bridged by Ca2+ is 2.92Å, which is in close agreement with the 2.8Å reported from X-ray diffraction measurements (16). These findings provide new insights into our understanding of the chemistry of membrane fusion in cells.
Secretory vesicle volume increase, and regulated content expulsion during cell secretion: In the early 1990s it was reported that secretory vesicles undergo an increase in volume during cell secretion (40,41). However, the molecular mechanism underlying volume regulation of secretory vesicles, and the role of this volume increase on secretory vesicle function during cell secretion, was poorly understood. Studies by the Jena laboratory showed that water channels or aquaporin in conjunction with several ion channels present at the secretory vesicle membrane regulate vesicle volume through GTP-binding G-proteins (24-30). The role of various ion channels at the secretory vesicle membrane was also demonstrated using single vesicle patch studies (42). In 2004, Jena and his research team reported that secretory vesicle volume increase is a requirement for the regulated release of intra-vesicular contents from cells (26). The relative increase in vesicle volume during cell secretion is proportional to the fraction of the intra-vesicular contents expelled.
In summary, studies by the Jena laboratory in the past over 25 years has demonstrated the presence of new cup-shaped lipoprotein structures at the cell plasma membrane called porosomes—the universal secretory machinery in cells—and elucidated the involvement of the porosome in the regulate fractional release of intra-vesicular contents from cells with exquisite precision involving membrane fusion and secretory vesicle volume regulation. Jena and his research team have isolated the porosome from a number of secretory cells including neurons, determined its composition, functionally reconstituted it in lipid membrane and live cells, and determined its dynamics and high-resolution structure using a variety of approaches including AFM, EM, and SAXS. Complementing the regulation of the porosome function, their studies have further contributed to our understanding of membrane fusion and secretory vesicle volume regulation, both required for the regulated fractional release of intra-vesicular contents during cell secretion. These results provide for the first time a molecular understanding of the regulated fractional release of intra-vesicular contents from cells during secretion. Recent studies by the Jena laboratory using mass spectrometry, demonstrate interaction between the cystic fibrosis trans-membrane conductance regulator (CFTR) and the porosome complex in human airways epithelia, shedding light on the possible regulatory role of CFTR on the quality of mucus secretion via the porosome complex (43). Results from this study provide critical insights into the etiology of CF disease and for potential therapies. Similarly, their recent studies using mass spectrometry provide understanding of the lipidome of the neuronal porosome complex (44), and the role of Hsp90 in porosome assembly and structure-function (45, 46). To determine the distribution of proteins within the porosome complex, the Jena laboratory is currently engaged in single particle electron crystallography, cryo-electron microscopy, X-ray solution and neutron scattering, and chemical cross linking followed by mass spectrometry. In a recent publication (10), they report for the first time the functional reconstitution of the insulin-secretion porosome complex into live insulin secreting cells, resulting in an increase in the potency and efficacy of glucose-stimulated insulin release, and a promise for therapeutic applications. In the past 15-years, there have been scores of publication by other laboratories on the porosome complex and the involvement of various porosome proteins in secretion and in secretory defects, some referenced here (37,47–62), attesting to the critical role played by the porosome in health and disease.
Reflecting on the Porosome Discovery
In the past 400 years since the invention of the light microscope, which led to the discovery that the unit of life the cell, we have witnessed an explosion in our understanding of life. This new understanding has taught us that Nature is the ultimate architect and engineer, capable of designing and building the most effective and efficient living unit the “Cell” and the precision nanomachines within, to undertake various life functions. Cellular nanomachines are a marvel of Nature, undertaking with great precision the vital cellular tasks like synthesis of new proteins life’s building blocks to their proper folding and assembly, their transport, and their secretion from within the cell to the outside. Secretion is required for cell–cell communication such as neurotransmission for coordination, thought, memory, smell, vision, taste, and hearing, for the digestion of food, for endocrine control such as insulin release in response to elevated blood glucose, and for release of immune products in response to a foreign antigen such as a pathogenic bacteria or virus, among others.
Each discovery of a nanomachine—such as the proteasome or cell’s garbage disposal; chaperonin, the protein folding machinery in cells; the ribosome, the protein synthetic machinery in cells; the signal recognition particle, the address tag in cells for protein targeting, and the porosome, -the secretory machinery in cells, all central to life, health and disease, are a gift to science and humanity.
The porosome discovery by Jena and his group and his international collaborators, and the data from other laboratories, are incontrovertible, that such a structure exists and is important for life processes. Moreover, the porosome comprises well-known proteins—there is nothing magical here—but these well-known proteins assemble in a beautiful and highly functional way that no one would have imagined -a major paradigm shift. However, a problem with paradigm shifts is that (as Arthur Schopenhauer (1788-1860) so elegantly put it: there are three stages of Truth: (a) First it is ridiculed, (b) Then violently opposed, and (c) Finally accepted as self-evident. Images of the native porosome complex, is right there in front of us to marvel in Nature’s beauty !
References:
- Journal of Cell Biology 1973, 57:499-524.
- Neuron 1990, 4:813-818.
- Journal of Cell Biology 1992,119:1395-1404.
- Proc. Natl. Acad. Sci, USA1997, 94:316-321.
- Cell Biol. Int 2002, 26(1):35-42.
- Biophys. J. 2003, 84(2):1-7.
- Endocrinology 2002, 143(3):1144-1148.
- New York Academy of Science Annals 2002, 971:254-256.
- Biophys. J. 2003, 85:2035-2043.
- Endocrinology 2016, 157:54-60.
- Cell Biol. Int. 2004, 28:699-708.
- Journal of Proteomics 2012, 75:3952-3962.
- Micron 2014, 56:37-43.
- Springer Briefs in Biological Imaging 2012, 1:1-70.
- Biophys. J. 2002, 83(5):2522-2527.
- Cell Biol. Int. 2004, 28:19-31.
- J. Biol. Phys. & Chem. 2004, 4:139-142.
- J. Am. Chem. Soc. 2005, 127:10156-10157.
- J. Am. Chem. Soc. 2006, 128:26-27.
- Chem. Phys. Lett. 2008, 462:6-9.
- J. Am. Chem. Soc. 2010, 132:5596-5597.
- J. Phys. Chem. 2010, 114:13249-13254.
- J. Cell. Mol. Med. 2011, 15:31-37.
- Proc. Natl. Acad. Sci, USA. 1997, 94:13317-13322.
- Proc. Natl. Acad. Sci. USA. 2002, 99(7):4720-4724.
- Cell Biol. Int. 2004, 28:709-716.
- Exp. Biol. Med. 2005, 230:674-680.
- J. Neurosci. Res. 2010, 88(1):95-101.
- Exp. Biol. Med. 2010, 235:470-477.
- J. Cell. Mol. Med. 2011, 15:572-576.
- Proc. Natl. Acad. Sci. USA. 1988, 85:4538-4542.
- Science 1992, 257:255-259.
- J. Cell Biol. 1989, 109:3039-3052.
- Nature 1998, 395:347–353.
- Cell 1998, 92:759-772.
- J. Cell Sci. 2010, 123:3276-3283.
- J. R. Soc. Interface. 2013;10:20130640. doi: 10.1098/rsif.2013.0640
- Biochemistry 1979, 18:780-790.
- Cell Biol. Int. 2008, 32:361-366.
- Nature 1991, 363:554–558.
- Biophys J. 1991, 59:39–47.
- Pancreatology 2005, 5:443-449.
- Journal of Proteomics 2013, 96:82-91.
- J. Cell. Mol. Med. 2014, 18:1927-1937.
- Journal of Proteomics 2015, 114:83-92.
- J. Mol. Struct. 2014, 1073:187-195.
- Cell Biol. Int. Rep. 2011, 18:31-34.
- J. Phys. Chem. B. 2010, 114:5971-5982.
- J. Am. Sci. 2011, 7:835-843.
- Micron 2013, 44:137-142.
- Journal of Physiology 2007, 579:85-99.
- Neuroscience 2009, 162:383-395.
- Journal of Biological Chemistry 2001, 276:17584-17590.
- Journal of Physiology 2000, 525(3):579-586.
- Journal of biological chemistry 2013, 288:20966-20977.
- Brain research 1999, 838:37-44.
- European journal of neuroscience 2007, 25:59-167.
- Electrophoresis 1999, 20:928-934.
- Cerebral cortex 2014, 24:364-376.
- Journal of neurochemistry 2010, 112:991-1004.
- Neuron 1999, 23:593-605.
- The Scientific World Journal 2009, 9:1463-1475
| Brief Narrative of Porosome Discovery: | Narrative on Contributions of Jena & Lab.pdf |
